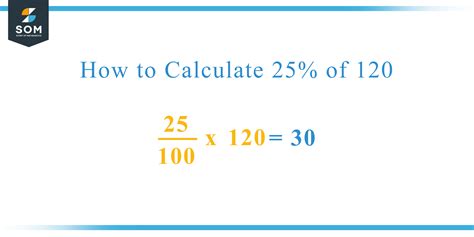The Empirical Formula Calculator Tool is a vital resource for chemists and researchers, enabling the determination of the simplest whole-number ratio of atoms in a molecule. This tool is based on the empirical formula, which is the simplest expression of a compound's composition, providing a foundation for understanding the molecular structure and properties of substances. The empirical formula is a fundamental concept in chemistry, and its calculation is a crucial step in the identification and characterization of compounds.
Understanding Empirical Formulas
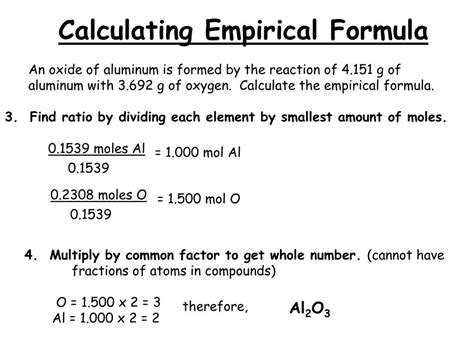
Empirical formulas are calculated from the percentage composition of a compound, which is typically obtained through experimental methods such as combustion analysis or mass spectrometry. The percentage composition provides the relative amounts of each element present in the compound, and the empirical formula is derived by dividing each percentage by the atomic mass of the corresponding element and then simplifying the resulting ratio to the simplest whole-number ratio. This process involves a series of calculations, including converting percentages to masses, dividing by atomic masses, and simplifying the resulting ratio.
Empirical Formula Calculation Steps
The calculation of an empirical formula involves several steps, including:
- Converting the percentage composition to masses of each element
- Dividing each mass by the atomic mass of the corresponding element
- Simplifying the resulting ratio to the simplest whole-number ratio
For example, if a compound has a percentage composition of 40.0% carbon, 6.7% hydrogen, and 53.3% oxygen, the empirical formula can be calculated as follows:
| Element | Percentage Composition | Atomic Mass | Mass | Ratio |
|---|---|---|---|---|
| Carbon | 40.0% | 12.01 g/mol | 40.0 g / 12.01 g/mol = 3.33 mol | 3.33 |
| Hydrogen | 6.7% | 1.01 g/mol | 6.7 g / 1.01 g/mol = 6.63 mol | 6.63 |
| Oxygen | 53.3% | 16.00 g/mol | 53.3 g / 16.00 g/mol = 3.33 mol | 3.33 |
Dividing each ratio by the smallest ratio (3.33) gives a simplified ratio of 1:2:1, which corresponds to an empirical formula of CH₂O.
Key Points
- The empirical formula is the simplest whole-number ratio of atoms in a molecule
- The empirical formula is calculated from the percentage composition of a compound
- The calculation involves converting percentages to masses, dividing by atomic masses, and simplifying the resulting ratio
- The empirical formula provides a foundation for understanding the molecular structure and properties of substances
- The Empirical Formula Calculator Tool is a vital resource for chemists and researchers
Applications of Empirical Formulas
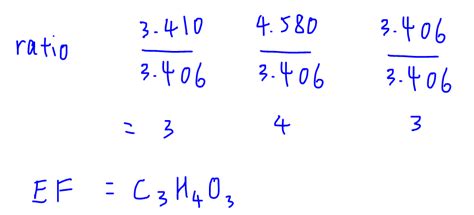
Empirical formulas have numerous applications in chemistry and related fields, including:
- Identification and characterization of compounds
- Determination of molecular formulas
- Calculation of molecular weights
- Prediction of physical and chemical properties
For example, the empirical formula of a compound can be used to predict its molecular weight, which is essential for understanding its physical and chemical properties. The empirical formula can also be used to determine the molecular formula, which provides a more detailed description of the molecular structure.
Limitations and Considerations
While empirical formulas are a powerful tool for understanding the composition and properties of substances, there are several limitations and considerations that must be taken into account. For example:
- Empirical formulas do not provide information about the molecular structure or arrangement of atoms
- Empirical formulas may not be unique, as different compounds can have the same empirical formula
- Empirical formulas are sensitive to experimental errors and uncertainties in the percentage composition
Therefore, it is essential to consider these limitations and use empirical formulas in conjunction with other analytical techniques, such as spectroscopy or chromatography, to obtain a more complete understanding of the composition and properties of substances.
What is the empirical formula, and how is it calculated?
+The empirical formula is the simplest whole-number ratio of atoms in a molecule, calculated from the percentage composition of a compound. The calculation involves converting percentages to masses, dividing by atomic masses, and simplifying the resulting ratio.
What are the applications of empirical formulas in chemistry?
+Empirical formulas have numerous applications in chemistry, including identification and characterization of compounds, determination of molecular formulas, calculation of molecular weights, and prediction of physical and chemical properties.
What are the limitations and considerations of empirical formulas?
+Empirical formulas have several limitations and considerations, including the lack of information about molecular structure, potential non-uniqueness, and sensitivity to experimental errors and uncertainties in the percentage composition.
Meta description suggestion: “Calculate the empirical formula of a compound using the Empirical Formula Calculator Tool. Learn about the applications, limitations, and considerations of empirical formulas in chemistry.” (147 characters)