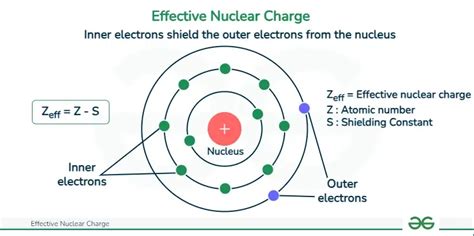
The concept of nuclear charge is a fundamental aspect of nuclear physics, playing a crucial role in understanding the behavior of atomic nuclei. It is defined as the total positive charge carried by the protons present in an atomic nucleus. The nuclear charge of an atom is denoted by the symbol Z and is measured in units of elementary charge (e). Understanding how nuclear charge works is essential for grasping various phenomena in physics and chemistry, including the structure of atoms, chemical bonding, and nuclear reactions. In this article, we will delve into five key ways nuclear charge influences the properties and behaviors of atoms and their interactions.
Introduction to Nuclear Charge and Its Role in Atomic Structure

Nuclear charge is a critical component of an atom’s structure, influencing its chemical properties and reactivity. The number of protons in an atom’s nucleus, which determines its nuclear charge, is also equal to the number of electrons in a neutral atom. This balance between protons and electrons is what gives an atom its chemical identity. For instance, an atom with a nuclear charge of +6 is carbon, and it will have 6 electrons in its neutral state. This intrinsic property of atoms is foundational to the periodic table, where elements are arranged based on their atomic number (which is the same as the nuclear charge) and exhibit periodic trends in their chemical properties.
Key Points
- The nuclear charge of an atom is determined by the number of protons in its nucleus.
- Nuclear charge influences the chemical properties and reactivity of an element.
- The balance between protons and electrons in a neutral atom is crucial for its chemical identity.
- Nuclear charge is fundamental to understanding the structure of the periodic table.
- It plays a role in determining the electron configuration and, consequently, the chemical behavior of an element.
Influence on Electron Configuration and Chemical Behavior
The nuclear charge has a profound effect on the electron configuration of an atom. According to the atomic model, electrons are arranged in shells or energy levels around the nucleus. The positive nuclear charge attracts electrons towards the nucleus, and the strength of this attraction increases with the nuclear charge. This means that as you move across the periodic table from left to right, the electrons in the outermost shell experience a stronger pull towards the nucleus due to the increasing nuclear charge. This influence on electron configuration, in turn, affects the chemical behavior of an element, including its ability to form ions, its reactivity, and the types of chemical bonds it can form.
| Element | Nuclear Charge | Electron Configuration |
|---|---|---|
| Hydrogen | +1 | 1s¹ |
| Helium | +2 | 1s² |
| Lithium | +3 | [He] 2s¹ |
| Boron | +5 | [He] 2s² 2p¹ |

Nuclear Charge and Nuclear Stability
The nuclear charge also plays a crucial role in determining the stability of an atomic nucleus. The strong nuclear force, which holds protons and neutrons together in the nucleus, must overcome the electrostatic repulsion between the positively charged protons. As the nuclear charge increases, so does the electrostatic repulsion between protons, which can lead to instability in the nucleus. However, the presence of neutrons, which have no charge, helps to stabilize the nucleus by contributing to the strong nuclear force without adding to the electrostatic repulsion. The balance between protons and neutrons is critical for nuclear stability, with certain ratios of neutrons to protons leading to more stable nuclei.
Role in Chemical Bonding
Nuclear charge is essential for understanding the formation of chemical bonds. The ability of an atom to attract electrons towards itself, known as electronegativity, is directly related to its nuclear charge. Atoms with a high nuclear charge tend to have higher electronegativity because they attract electrons more strongly. This attraction is fundamental to the formation of ionic bonds, where one or more electrons are transferred from an atom with a low nuclear charge (and thus lower electronegativity) to an atom with a higher nuclear charge (and higher electronegativity), resulting in the formation of ions that are electrostatically attracted to each other. Covalent bonds also involve the sharing of electrons between atoms, and the nuclear charge influences the polarity of these bonds, with atoms of higher nuclear charge pulling the shared electrons closer to themselves.
Implications for Nuclear Reactions
Nuclear charge has significant implications for nuclear reactions, including fission and fusion. In nuclear fission, an atomic nucleus splits into two or more smaller nuclei, along with the release of energy, neutrons, and gamma radiation. The feasibility of fission depends on the nuclear charge and the number of neutrons in the nucleus. Certain isotopes, like uranium-235, are fissile because their nuclei can be split by neutron bombardment, releasing more neutrons that can cause subsequent fission reactions. Nuclear fusion, on the other hand, involves the combination of two light nuclei to form a heavier nucleus, releasing energy in the process. Achieving and sustaining fusion reactions requires overcoming the electrostatic repulsion between the positively charged nuclei, which is directly related to their nuclear charges.
What is nuclear charge, and how is it measured?
+Nuclear charge refers to the total positive charge of an atomic nucleus and is measured by the number of protons it contains, denoted by the atomic number Z.
How does nuclear charge affect the chemical properties of an element?
+The nuclear charge influences the electron configuration of an atom, which in turn affects its chemical properties, including reactivity and the ability to form bonds with other atoms.
What role does nuclear charge play in nuclear stability and reactions?
+Nuclear charge is critical for nuclear stability, as it must be balanced with the number of neutrons to prevent electrostatic repulsion from overpowering the strong nuclear force. It also plays a role in determining the feasibility of nuclear reactions like fission and fusion.
In conclusion, nuclear charge is a fundamental property of atoms that underlies many of the chemical and physical phenomena we observe. Its influence on electron configuration, chemical bonding, nuclear stability, and nuclear reactions makes it a cornerstone of modern physics and chemistry. Understanding nuclear charge and its effects is essential for advancing our knowledge of the atomic world and harnessing its potential for technological and scientific progress.