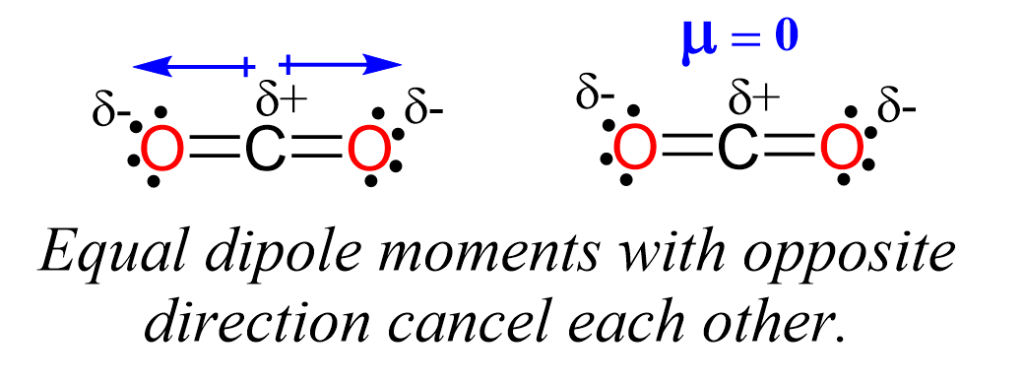
The question of whether CO2 (carbon dioxide) is polar or nonpolar is a fundamental concept in chemistry, particularly in understanding the properties and behaviors of molecules. To address this, we first need to understand what it means for a molecule to be polar or nonpolar. A polar molecule is one that has a net dipole moment, meaning it has a slightly positive charge on one side and a slightly negative charge on the other due to the difference in electronegativity between the atoms in the molecule. On the other hand, a nonpolar molecule has no net dipole moment, as the charges are evenly distributed.
Understanding CO2 Molecular Structure
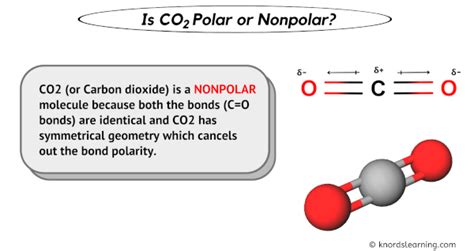
CO2 consists of one carbon atom covalently bonded to two oxygen atoms. The carbon atom is in the center, and the two oxygen atoms are on either side, forming a linear molecule (O=C=O). The bond between carbon and oxygen is a double bond, which is highly stable. In this linear configuration, the carbon-oxygen bonds are symmetrical, which is crucial for determining the polarity of the molecule.
Electronegativity and Bond Polarity
Oxygen is more electronegative than carbon, meaning it has a greater tendency to attract electrons. In a carbon-oxygen bond, the oxygen atom pulls the shared electrons closer to itself, resulting in a partial negative charge on the oxygen and a partial positive charge on the carbon. However, because the CO2 molecule is linear and symmetrical, the pulls of the two oxygen atoms on the carbon atom are equal and in opposite directions.
| Atom | Electronegativity |
|---|---|
| Carbon (C) | 2.55 |
| Oxygen (O) | 3.44 |
Conclusion on Polarity

Given the linear and symmetrical structure of CO2, where the two oxygen atoms are equally distant from the carbon atom and pull with equal force in opposite directions, the molecule does not have a net dipole moment. Therefore, CO2 is considered a nonpolar molecule. This characteristic influences many of its physical and chemical properties, such as its solubility in water and its ability to dissolve other nonpolar substances.
Key Points
- CO2 has a linear molecular structure with a carbon atom bonded to two oxygen atoms.
- The carbon-oxygen bonds are polar due to the difference in electronegativity between carbon and oxygen.
- The symmetry of the CO2 molecule means the polar effects of the carbon-oxygen bonds cancel out, resulting in no net dipole moment.
- CO2 is classified as a nonpolar molecule due to its lack of a net dipole moment.
- The nonpolarity of CO2 affects its physical and chemical properties, including its solubility and interactions with other molecules.
Implications and Applications
Understanding whether CO2 is polar or nonpolar is crucial for various applications, including environmental science, where the properties of CO2 influence its behavior in the atmosphere and its interactions with other molecules. In industrial processes, knowing the polarity of CO2 can inform the design of systems for capturing, storing, or utilizing CO2, such as in carbon capture and storage (CCS) technologies or in the production of chemicals and fuels.
What makes a molecule polar or nonpolar?
+A molecule is polar if it has a net dipole moment, resulting from an asymmetrical distribution of charge. It is nonpolar if it has no net dipole moment, often due to a symmetrical molecular structure.
Why is the polarity of CO2 important?
+The polarity of CO2 influences its physical and chemical properties, such as solubility, boiling point, and reactivity, which are crucial for understanding its behavior in various environments and applications.
Can CO2’s polarity affect its environmental impact?
+Yes, the nonpolarity of CO2 contributes to its ability to mix with other nonpolar substances and its low reactivity with water, which can influence its transport and fate in the environment, including its role in climate change.