The molecular geometry of carbon dioxide, commonly referred to as CO2, is a fundamental concept in chemistry that has significant implications for our understanding of the natural world and the impact of human activities on the environment. At its core, the study of CO2 molecular geometry involves the arrangement of atoms within the molecule and how this arrangement influences its physical and chemical properties. To delve into the specifics of CO2's molecular geometry, it is essential to first understand the basic principles of molecular structure and the factors that determine the shape of a molecule.
Carbon dioxide is composed of one carbon atom and two oxygen atoms, with the chemical formula CO2. The carbon atom, being the central atom, is bonded to the two oxygen atoms through covalent bonds. The nature of these bonds and the electron geometry around the central carbon atom play a crucial role in determining the molecular geometry of CO2. According to the VSEPR (Valence Shell Electron Pair Repulsion) theory, which is a model used to predict the shape of molecules, the shape of a molecule is determined by the repulsion between electron pairs in the valence shell of the central atom.
Electron Geometry and Molecular Shape
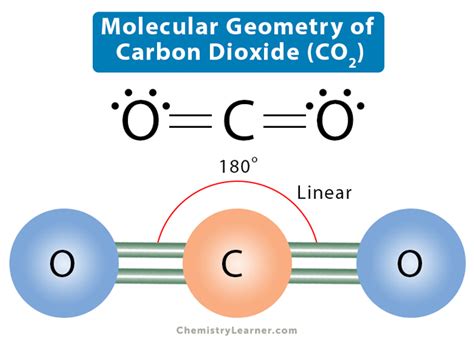
In the case of CO2, the carbon atom is sp-hybridized, meaning it has two pairs of electrons in its valence shell that are involved in bonding with the oxygen atoms, and no lone pairs. This results in a linear electron geometry around the carbon atom, as the two electron pairs (each shared with an oxygen atom) are arranged as far apart as possible to minimize repulsion. Since there are no lone pairs on the central atom, the molecular geometry of CO2 is also linear, with the carbon atom situated at the center and the two oxygen atoms at opposite ends.
Key Characteristics of CO2 Molecular Geometry
The linear molecular geometry of CO2 has several key characteristics. Firstly, the molecule has a symmetrical shape, with the two CO bonds being of equal length (approximately 116 picometers) and the OCO angle being 180 degrees. This symmetry contributes to the molecule's lack of a permanent electric dipole moment, making CO2 a non-polar molecule despite the difference in electronegativity between carbon and oxygen atoms. Secondly, the linear shape of CO2 allows it to pack efficiently in the solid state, contributing to its relatively high density when compared to other gases.
| Property | Value |
|---|---|
| Bond Length (CO) | 116 pm |
| Bond Angle (OCO) | 180 degrees |
| Molecular Weight | 44.01 g/mol |
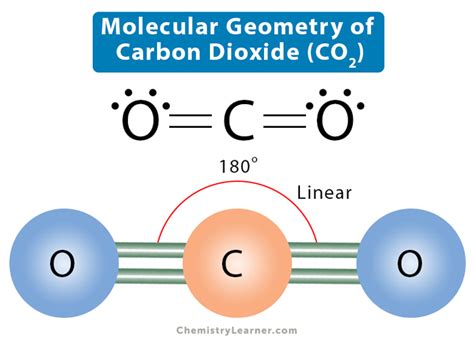
The molecular geometry of CO2 also has implications for its chemical reactivity. The linear shape and the lack of lone pairs on the central carbon atom make CO2 an electrophile, capable of reacting with nucleophiles such as hydroxide ions to form bicarbonate. This reactivity is vital in many biological and industrial processes, including photosynthesis and the production of carbonated beverages.
Environmental Implications

The increase in CO2 concentrations in the Earth's atmosphere, primarily due to fossil fuel combustion and deforestation, has significant environmental implications. The enhanced greenhouse effect resulting from increased CO2 levels leads to global warming and associated climate change impacts, including rising sea levels, more extreme weather events, and alterations to ecosystems. Understanding the molecular geometry of CO2 and its implications for its physical and chemical properties is essential for developing strategies to mitigate and adapt to these changes.
Key Points
- The molecular geometry of CO2 is linear, resulting from the sp-hybridization of the carbon atom and the absence of lone pairs.
- The linear shape of CO2 contributes to its non-polar nature and efficient packing in the solid state.
- The molecular geometry of CO2 is crucial for its role as a greenhouse gas and its reactivity in various chemical processes.
- Understanding CO2's molecular geometry provides insights into its behavior and implications for environmental processes.
- The increase in CO2 concentrations in the atmosphere has significant environmental implications, including global warming and climate change.
In conclusion, the molecular geometry of CO2, characterized by its linear shape, plays a pivotal role in its physical and chemical properties, as well as its environmental implications. As we continue to navigate the challenges posed by climate change, a deep understanding of the molecular geometry of CO2 and its effects on our planet will be indispensable for developing effective strategies to mitigate these impacts and ensure a sustainable future.
What is the significance of CO2’s linear molecular geometry?
+The linear molecular geometry of CO2 is significant because it contributes to the molecule’s non-polar nature and its ability to efficiently pack in the solid state. This geometry also plays a crucial role in CO2’s ability to absorb and emit infrared radiation, making it a significant greenhouse gas.
How does the molecular geometry of CO2 influence its reactivity?
+The linear geometry and the lack of lone pairs on the central carbon atom make CO2 an electrophile, capable of reacting with nucleophiles. This reactivity is vital in many biological and industrial processes, including photosynthesis and the production of carbonated beverages.
What are the environmental implications of increased CO2 concentrations in the atmosphere?
+The increase in CO2 concentrations leads to the enhanced greenhouse effect, resulting in global warming and associated climate change impacts, including rising sea levels, more extreme weather events, and alterations to ecosystems.