Chemical nomenclature, the systematic method of naming chemical compounds, is a fundamental aspect of chemistry that often poses a significant challenge for students and professionals alike. The International Union of Pure and Applied Chemistry (IUPAC) has established a set of rules to ensure that chemical compounds are named consistently and unambiguously. Mastering chemical nomenclature is essential for effective communication among chemists, as it allows for the precise identification and description of substances. In this article, we will delve into the world of chemical nomenclature, exploring its principles, rules, and applications, to make practice easier and more accessible.
Key Points
- Understanding the basics of IUPAC nomenclature is crucial for naming organic and inorganic compounds.
- Prefixes, suffixes, and infixes play a vital role in constructing chemical names.
- Recognizing functional groups and their corresponding suffixes is essential for naming organic compounds.
- Practice and repetition are key to becoming proficient in chemical nomenclature.
- Utilizing online resources and nomenclature tools can facilitate the learning process.
Introduction to IUPAC Nomenclature
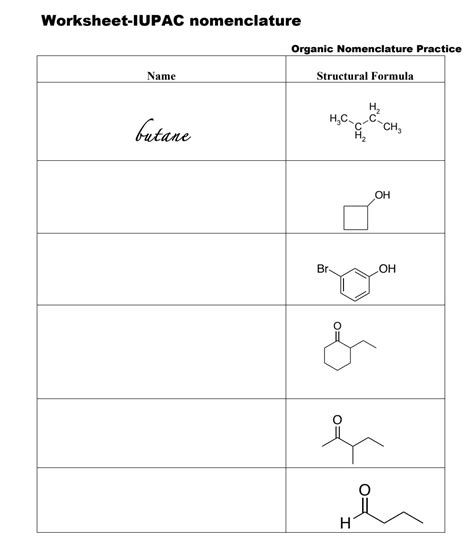
The IUPAC system of nomenclature is based on a set of rules that provide a logical and consistent approach to naming chemical compounds. The system is divided into two main categories: organic and inorganic nomenclature. Organic compounds are typically named using a combination of prefixes, suffixes, and infixes, while inorganic compounds are named using a more straightforward approach based on their composition. To illustrate this, consider the example of ethanol, which is named using the prefix “eth-” to indicate the presence of two carbon atoms, and the suffix “-ol” to indicate the presence of a hydroxyl functional group.
Organic Nomenclature
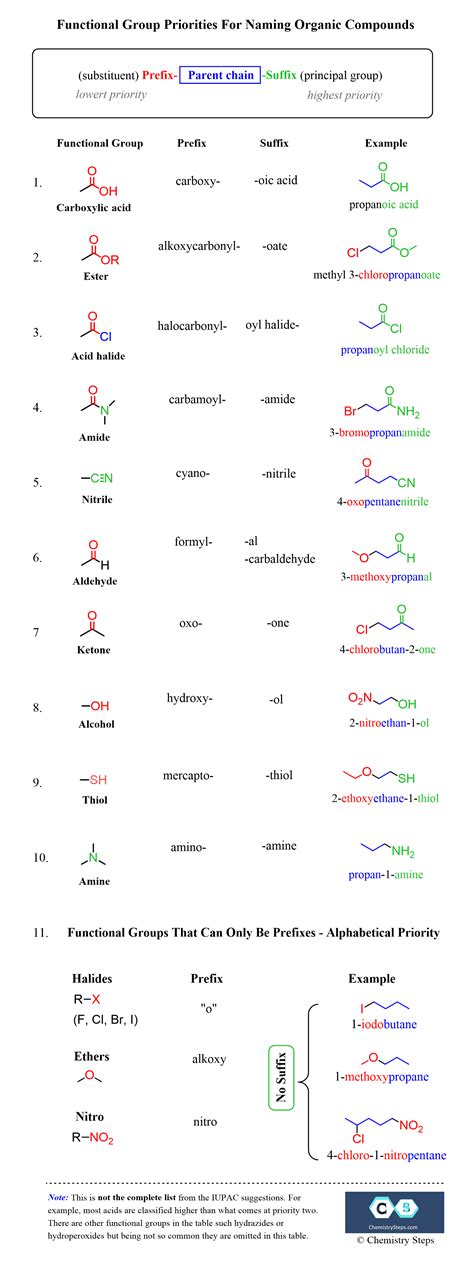
Organic nomenclature is the most complex and challenging aspect of chemical nomenclature. It involves the use of prefixes, suffixes, and infixes to describe the structure and functionality of a molecule. Prefixes are used to indicate the presence of specific functional groups, such as alkyl or aryl groups, while suffixes are used to indicate the type of compound, such as an alkane or an alkene. Infixes are used to indicate the location of functional groups or substituents within the molecule. For instance, the compound 2-methylpropane is named using the prefix “methyl-” to indicate the presence of a methyl group, and the suffix “-ane” to indicate that it is an alkane.
| Functional Group | Suffix |
|---|---|
| Alkane | -ane |
| Alkene | -ene |
| Alkyne | -yne |
| Alcohol | -ol |
| Amine | -amine |
Inorganic Nomenclature
Inorganic nomenclature is relatively simpler than organic nomenclature. It involves the use of a straightforward approach based on the composition of the compound. Inorganic compounds are typically named using a combination of the cation and anion names, with the cation name first, followed by the anion name. For example, sodium chloride is named by combining the cation name “sodium” with the anion name “chloride”.
Acid Nomenclature
Acid nomenclature is a specialized aspect of inorganic nomenclature. Acids are named using a combination of the anion name and the suffix “-ic acid” or “-ous acid”. For example, sulfuric acid is named using the anion name “sulfate” and the suffix “-ic acid”.
| Anion | Acid Name |
|---|---|
| Sulfate | Sulfuric acid |
| Nitrate | Nitric acid |
| Chloride | Hydrochloric acid |
Mastering chemical nomenclature requires practice, patience, and dedication. By understanding the principles and rules of IUPAC nomenclature, you can develop a systematic approach to naming chemical compounds. Remember to focus on recognizing functional groups, using prefixes, suffixes, and infixes correctly, and practicing with a variety of examples. With time and effort, you'll become proficient in chemical nomenclature and be able to communicate effectively with other chemists.
What is the primary purpose of IUPAC nomenclature?
+The primary purpose of IUPAC nomenclature is to provide a systematic and consistent approach to naming chemical compounds, ensuring that each compound has a unique and unambiguous name.
How do I determine the suffix for an organic compound?
+The suffix for an organic compound is determined by the functional group present in the molecule. For example, an alkane has the suffix "-ane", while an alkene has the suffix "-ene".
What is the difference between inorganic and organic nomenclature?
+Inorganic nomenclature is relatively simpler and involves the use of a straightforward approach based on the composition of the compound, while organic nomenclature is more complex and involves the use of prefixes, suffixes, and infixes to describe the structure and functionality of a molecule.
Meta Description: Master chemical nomenclature with ease using our comprehensive guide to IUPAC rules and practices. Learn to name organic and inorganic compounds with confidence. (149 characters)