The carbonate ion, with the chemical formula CO3^2-, is a fundamental component in various chemical and biological processes. Understanding the charge on the carbonate ion is essential for grasping its role in these processes. The carbonate ion is formed when carbonic acid (H2CO3) loses two protons (H+), resulting in a molecule with a net negative charge. This negative charge is a result of the difference between the number of electrons and protons in the ion. In the carbonate ion, there are 24 electrons (6 from carbon, 18 from the three oxygen atoms) and 22 protons (6 from carbon, 8 from the two single-bonded oxygen atoms, and 8 from the double-bonded oxygen atom, but since two protons are lost, we subtract 2), leading to a net charge of -2.
Key Points
- The carbonate ion has a chemical formula of CO3^2- and a net negative charge of -2.
- The charge on the carbonate ion is due to the loss of two protons from carbonic acid.
- Understanding the charge on the carbonate ion is crucial for comprehending its role in various chemical and biological processes.
- The carbonate ion plays a significant role in the carbon cycle and in the formation of shells and skeletons of marine organisms.
- The charge on the carbonate ion influences its reactivity and interactions with other ions and molecules.
Chemical Structure and Charge Distribution
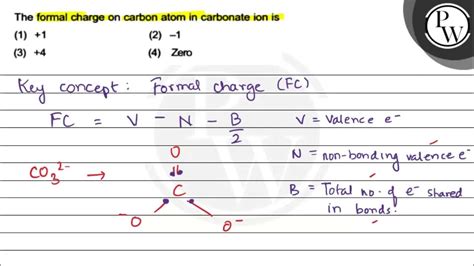
The chemical structure of the carbonate ion consists of a central carbon atom bonded to three oxygen atoms. Two of the oxygen atoms are single-bonded to the carbon, while the third oxygen atom is double-bonded. The double bond is delocalized across the ion, resulting in a resonance structure that distributes the negative charge evenly across the three oxygen atoms. This delocalization of the charge contributes to the stability of the carbonate ion and influences its chemical properties.
Formation and Stability
The carbonate ion is formed through the dissociation of carbonic acid (H2CO3) in water. This process involves the loss of two protons, resulting in the formation of the carbonate ion (CO3^2-) and two hydrogen ions (H+). The stability of the carbonate ion is influenced by the pH of the solution, with the ion being more stable at higher pH values. The charge on the carbonate ion also plays a role in its stability, as it affects the ion’s ability to interact with other ions and molecules in the solution.
| Property | Value |
|---|---|
| Chemical Formula | CO3^2- |
| Net Charge | -2 |
| pH Range for Stability | 8-14 |
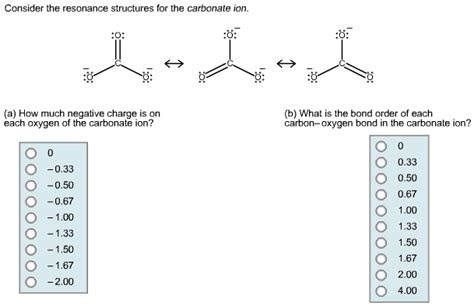
Biological and Chemical Significance
The carbonate ion plays a vital role in various biological and chemical processes. In the context of marine biology, the carbonate ion is essential for the formation of shells and skeletons of organisms such as corals, shellfish, and some plankton. The ion also participates in the carbon cycle, where it is involved in the conversion of carbon dioxide (CO2) into organic compounds. Furthermore, the carbonate ion is used in various industrial applications, including the production of glass, paper, and detergents.
Environmental Impact
The charge on the carbonate ion also has environmental implications. For instance, the increased levels of carbon dioxide (CO2) in the atmosphere can lead to ocean acidification, which affects the availability of carbonate ions for marine organisms. This, in turn, can have significant impacts on marine ecosystems and the organisms that depend on the carbonate ion for their survival.
What is the chemical formula of the carbonate ion?
+The chemical formula of the carbonate ion is CO3^2-.
What is the net charge on the carbonate ion?
+The net charge on the carbonate ion is -2.
What is the role of the carbonate ion in the formation of shells and skeletons in marine organisms?
+The carbonate ion is essential for the formation of shells and skeletons in marine organisms, as it binds to positively charged ions like calcium (Ca^2+) to form compounds like calcium carbonate (CaCO3).
In conclusion, the charge on the carbonate ion is a critical aspect of its chemical and biological properties. The negative charge of -2, resulting from the loss of two protons from carbonic acid, influences the ion’s stability, reactivity, and interactions with other ions and molecules. Understanding the charge on the carbonate ion is essential for grasping its role in various biological and chemical processes, including the formation of shells and skeletons in marine organisms, the carbon cycle, and industrial applications.