The charge of a chloride ion is a fundamental concept in chemistry, particularly in the study of ions and their interactions. An ion is an atom or molecule that has gained or lost electrons, resulting in a net positive or negative charge. Chloride ions, denoted by the chemical symbol Cl⁻, are formed when a chlorine atom loses an electron. This process of electron loss or gain is known as ionization. In the case of chlorine, which is a halogen, the atom readily loses one electron to achieve a stable electron configuration similar to that of the noble gas argon, thus forming a chloride ion with a charge of -1.
The charge on an ion is determined by the number of electrons gained or lost by the atom. Since a chlorine atom has 17 electrons and loses one to form a chloride ion, the resulting ion has 17 - 1 = 16 electrons. However, the atomic number of chlorine, which represents the number of protons in the nucleus, remains 17. The difference between the number of protons (17) and the number of electrons (16) in a chloride ion gives it a net charge of -1. This negative charge is what characterizes chloride ions and influences their chemical properties and behaviors.
Key Points
- The charge of a chloride ion is -1, resulting from the loss of one electron by a chlorine atom.
- Chloride ions are formed through the ionization process, where a chlorine atom achieves a stable electron configuration by losing an electron.
- The charge on an ion is determined by the difference between the number of protons and electrons, with chloride having 17 protons and 16 electrons.
- Understanding the charge of ions like chloride is crucial for predicting their chemical reactions and interactions with other ions and molecules.
- Chloride ions play significant roles in various biological and chemical processes, including the maintenance of fluid balance and the transmission of nerve impulses in living organisms.
Formation and Properties of Chloride Ions
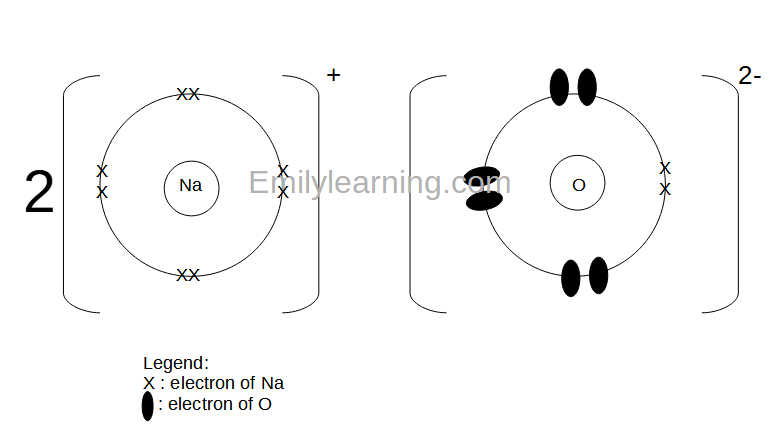
Chloride ions are commonly found in nature, particularly in seawater and mineral deposits. The formation of chloride ions can occur through various chemical reactions, including the reaction of chlorine gas with metals or the dissolution of chloride salts in water. For instance, when sodium chloride (NaCl), or common table salt, is dissolved in water, it dissociates into sodium ions (Na⁺) and chloride ions (Cl⁻). This dissociation is a key aspect of the chemical behavior of salts and is fundamental to understanding various biological and environmental processes.
Chemical Reactions Involving Chloride Ions
Chloride ions participate in a wide range of chemical reactions, including precipitation reactions, acid-base reactions, and oxidation-reduction reactions. In a precipitation reaction, for example, chloride ions can combine with other ions to form insoluble compounds. A well-known example is the reaction between chloride ions and silver ions (Ag⁺) to form silver chloride (AgCl), which is insoluble in water. This reaction is often used in qualitative analysis to detect the presence of chloride ions in a solution.
| Type of Reaction | Example | Products |
|---|---|---|
| Precipitation | Cl⁻ + Ag⁺ → AgCl | Silver chloride (solid) |
| Acid-Base | Cl⁻ + H⁺ → HCl | Hydrochloric acid |
| Oxidation-Reduction | 2Cl⁻ → Cl₂ + 2e⁻ | Chlorine gas, electrons |
Biological Importance of Chloride Ions
Chloride ions play crucial roles in living organisms, particularly in maintaining fluid balance and facilitating the transmission of nerve impulses. In humans, for instance, chloride ions are the most abundant type of anion in the extracellular fluid, which includes blood plasma. They help maintain the balance of fluids within the body and are involved in the proper functioning of the nervous system. The concentration of chloride ions in the body is tightly regulated, and abnormalities in chloride ion levels can lead to various health issues, including dehydration and neurological disorders.
Regulation of Chloride Ion Concentration
The concentration of chloride ions in the body is regulated through a complex system involving the kidneys, hormones, and various transport mechanisms across cell membranes. The kidneys play a key role by adjusting the amount of chloride ions excreted in the urine based on the body’s needs. Additionally, certain hormones can influence the reabsorption or excretion of chloride ions in the kidneys, thereby helping to maintain homeostasis.
In conclusion, the charge of a chloride ion, which is -1, is a fundamental aspect of its chemical properties and behaviors. Chloride ions are involved in a wide range of chemical reactions and play critical roles in biological processes. Understanding the properties and functions of chloride ions is essential for advancing knowledge in fields such as chemistry, biology, and medicine, and for addressing various environmental and health challenges.
What is the charge of a chloride ion?
+The charge of a chloride ion is -1, resulting from the loss of one electron by a chlorine atom.
How are chloride ions formed?
+Chloride ions are formed through the ionization process, where a chlorine atom loses an electron to achieve a stable electron configuration.
What roles do chloride ions play in biological processes?
+Chloride ions are crucial for maintaining fluid balance and facilitating the transmission of nerve impulses in living organisms.