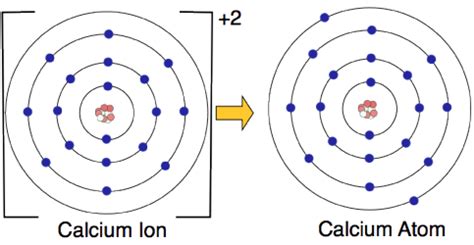
Calcium, an essential mineral for the human body, plays a critical role in various physiological processes, including muscle function, nerve transmission, and bone health. One of its key forms is the calcium ion, denoted as Ca²⁺, which is a positively charged ion that has lost two electrons to achieve a stable electronic configuration. The calcium ion is crucial for numerous biological processes, and its charge properties are fundamental to understanding its interactions within the body. In this article, we will delve into the world of calcium charge ions, exploring their properties, biological significance, and the importance of maintaining the right balance of calcium ions in the body.
Key Points
- The calcium ion (Ca²⁺) is a positively charged ion essential for various bodily functions.
- Calcium ions play a crucial role in muscle contraction, nerve impulse transmission, and bone mineralization.
- The balance of calcium ions in the body is tightly regulated, with hypercalcemia and hypocalcemia being conditions of excessive and deficient calcium levels, respectively.
- Dietary calcium intake is vital for maintaining adequate calcium levels, with dairy products, leafy greens, and fortified foods being significant sources.
- Calcium supplements can be beneficial for individuals with insufficient dietary intake or increased calcium requirements, such as during pregnancy or lactation.
Chemical Properties of Calcium Ions
Calcium ions have a +2 charge, resulting from the loss of two electrons from the neutral calcium atom. This charge is critical for its biological functions, as it allows calcium ions to interact with negatively charged molecules, such as phospholipids in cell membranes and the phosphate groups in nucleic acids. The size of the calcium ion, with a radius of approximately 0.99 Å (angstroms), also influences its interactions, particularly in the context of ionic channels and binding sites on proteins.
Biological Role of Calcium Ions
Calcium ions are involved in a myriad of biological processes, including muscle contraction, where they act as a trigger for the sliding filament mechanism. In the nervous system, calcium ions are essential for neurotransmitter release, facilitating communication between neurons. Additionally, calcium ions are crucial for bone health, as they are a key component of hydroxyapatite, the mineral that gives bones their strength and rigidity. The regulation of calcium ion concentrations is tightly controlled by the body, with hormones such as parathyroid hormone (PTH) and calcitriol (the active form of vitamin D) playing central roles.
| Biological Process | Role of Calcium Ions |
|---|---|
| Muscle Contraction | Trigger for sliding filament mechanism |
| Nerve Impulse Transmission | Essential for neurotransmitter release |
| Bone Mineralization | Component of hydroxyapatite, giving bones strength and rigidity |
Dietary Sources and Supplements
Dietary calcium is crucial for maintaining adequate levels of calcium ions in the body. Significant sources of calcium include dairy products (such as milk, cheese, and yogurt), leafy green vegetables (like broccoli, kale, and spinach), and fortified foods (such as cereals and plant-based milk alternatives). For individuals with insufficient dietary intake or increased calcium requirements, such as during pregnancy or lactation, calcium supplements can be beneficial. However, it is essential to consult with a healthcare provider before starting any supplement regimen to ensure the correct dosage and to avoid potential interactions with other medications.
Health Implications of Calcium Imbalance
Both hypercalcemia (excessive levels of calcium in the blood) and hypocalcemia (deficient levels of calcium) can have serious health implications. Hypercalcemia can lead to symptoms such as nausea, vomiting, and abdominal cramps, while severe cases can result in kidney stones, bone pain, and cardiac arrhythmias. Hypocalcemia, on the other hand, can cause muscle cramps, tingling in the fingers and around the mouth, and, in severe cases, tetany (a condition characterized by involuntary muscle contractions). Understanding the causes of these imbalances, such as vitamin D deficiency, hyperparathyroidism, or excessive calcium intake, is crucial for proper management and treatment.
What are the primary dietary sources of calcium?
+The primary dietary sources of calcium include dairy products, leafy green vegetables, and fortified foods. It is essential to maintain a balanced diet that includes a variety of these sources to ensure adequate calcium intake.
What are the symptoms of calcium deficiency?
+Symptoms of calcium deficiency (hypocalcemia) can include muscle cramps, tingling in the fingers and around the mouth, and, in severe cases, tetany. It is crucial to recognize these symptoms early to prevent more serious complications.
Can calcium supplements interact with other medications?
+Yes, calcium supplements can interact with certain medications, such as antibiotics and blood thinners. It is essential to consult with a healthcare provider before starting any supplement regimen to discuss potential interactions and ensure safe use.
In conclusion, calcium ions play a vital role in numerous biological processes, from muscle contraction and nerve impulse transmission to bone mineralization. Understanding the chemical properties, biological functions, and health implications of calcium ions is essential for maintaining optimal health. By recognizing the importance of dietary calcium, the potential need for supplements, and the risks associated with calcium imbalance, individuals can take proactive steps to ensure they are getting the right amount of calcium for their bodies.