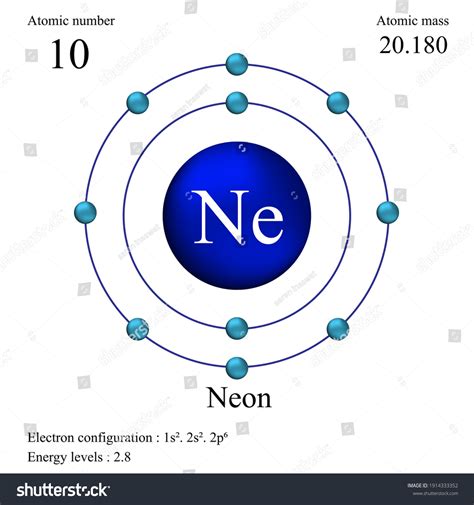
Neon, with the atomic number 10, is a noble gas that has been a cornerstone of various scientific and technological advancements. Its unique properties and characteristics have made it an essential element in fields such as lighting, lasers, and even cryogenics. To understand the significance of neon, it's crucial to delve into its atomic structure and how its atomic number influences its behavior and applications.
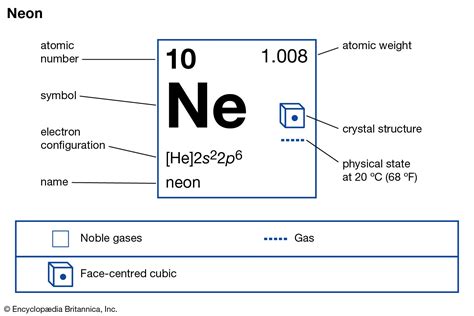
The atomic number of an element, which in this case is 10 for neon, indicates the number of protons present in the nucleus of an atom. This number is fundamental because it determines the element's position in the periodic table and its chemical properties. Neon, being a noble gas, has a full outer energy level, which makes it extremely stable and unreactive under normal conditions. This stability is a direct consequence of its atomic number and the resulting electron configuration.
Neon's discovery in 1898 by William Ramsay and Morris Travers marked the beginning of a new era in the understanding of noble gases. Initially, neon was considered a rare and exotic element, but its applications soon expanded beyond the scientific community. Today, neon is used in neon signs, plasma TVs, and even in the production of semiconductors. The element's unique glow when electrically excited, which is a result of its atomic structure, has made it a favorite for decorative and advertising purposes.
Key Points
- Neon's atomic number is 10, indicating it has 10 protons in its atomic nucleus.
- It is a noble gas with a full outer energy level, making it chemically stable and unreactive.
- Neon was discovered in 1898 by William Ramsay and Morris Travers.
- The element has various applications, including lighting, lasers, and semiconductor production.
- Neon's unique glow under electrical excitation makes it popular for decorative signs and displays.
Atomic Structure and Properties
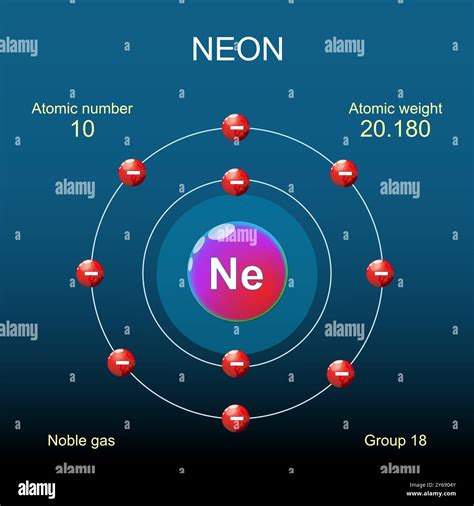
The atomic structure of neon is characterized by its atomic number of 10, which means it has 10 protons and 10 electrons in a neutral atom. The electron configuration of neon is 1s² 2s² 2p⁶, indicating a full outer shell that is responsible for its inertness. This stability is a key factor in neon’s applications, as it does not readily react with other elements to form compounds. The atomic mass of neon is approximately 20.1797 u (unified atomic mass units), which is a weighted average of its naturally occurring isotopes.
Isotopes and Occurrence
Neon has three stable isotopes: neon-20, neon-21, and neon-22. These isotopes occur naturally in the atmosphere, although in very small concentrations. Neon-20 is the most abundant, making up about 90.48% of natural neon, followed by neon-22 at 9.25%, and neon-21 at 0.27%. The isotopic composition of neon can vary slightly depending on the source, but these values are generally accepted. The occurrence of neon in the Earth’s atmosphere is a result of its presence in the solar system’s formation materials and its stability, which prevents it from reacting and being removed from the atmosphere.
| Isotope | Atomic Mass | Natural Abundance |
|---|---|---|
| Neon-20 | 19.9924358 u | 90.48% |
| Neon-21 | 20.9938468 u | 0.27% |
| Neon-22 | 21.9913851 u | 9.25% |
Applications and Uses
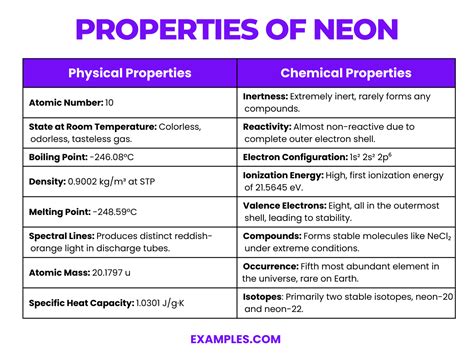
Neon’s unique properties have led to a wide range of applications. Its most recognizable use is in neon signs, where electrical excitation causes the gas to emit light. This property is also utilized in plasma TVs, where individual cells containing neon and other gases are electrically excited to produce images. Additionally, neon is used in lasers, where its ability to emit light at specific wavelengths is crucial for applications such as material cutting and spectroscopy.
Technological Advancements
The use of neon in technological advancements is not limited to lighting and displays. It is also an essential component in the production of semiconductors, where its inertness and stability are valuable. Neon is used as a doping gas to introduce impurities into silicon wafers, which is a critical step in creating the complex electronic circuits found in modern electronics. Furthermore, neon’s cooling properties make it useful in cryogenic applications, such as the cooling of superconducting materials and in cryogenic preservation techniques.
In conclusion, neon's atomic number of 10 is more than just a numerical identifier; it is the foundation of its chemical properties and applications. From its discovery to its current uses, neon has proven to be a versatile and essential element in both scientific research and technological innovation. Its unique glow, stability, and inertness have made it a cornerstone of various industries, ensuring its continued relevance and importance in the future.
What is the atomic number of neon and what does it signify?
+The atomic number of neon is 10, which signifies that a neon atom has 10 protons in its nucleus. This number determines neon’s position in the periodic table and its chemical properties.
What are the primary uses of neon?
+Neon is primarily used in lighting (neon signs), plasma TVs, lasers, and in the production of semiconductors. Its unique properties make it ideal for these applications.
Is neon a rare element?
+Neon is not considered a rare element in terms of its abundance in the universe, but it is rare in the Earth’s atmosphere, making up only a small fraction of the atmospheric gases.