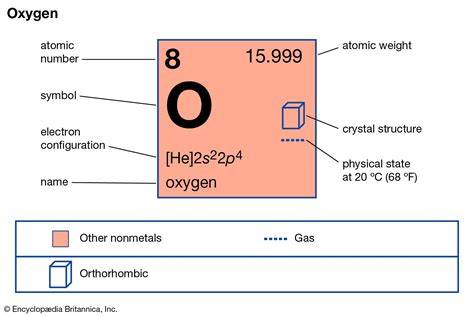
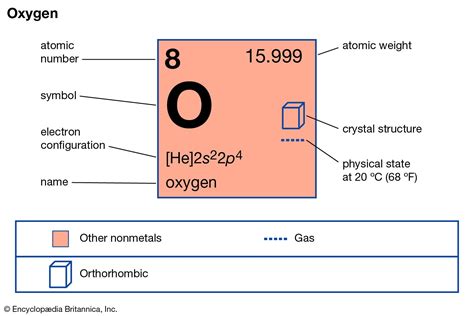
The atomic mass of oxygen is a fundamental concept in chemistry, and it plays a crucial role in understanding the properties and behavior of this essential element. Oxygen is a nonmetal that is highly reactive and is a major component of the Earth's atmosphere, making up approximately 21% of the air we breathe. The atomic mass of oxygen is 15.9994 u (unified atomic mass units), which is a weighted average of the masses of its naturally occurring isotopes.
Oxygen has three stable isotopes: oxygen-16, oxygen-17, and oxygen-18. The most abundant isotope is oxygen-16, which accounts for approximately 99.76% of natural oxygen. Oxygen-17 and oxygen-18 make up about 0.037% and 0.204% of natural oxygen, respectively. The atomic masses of these isotopes are 15.9949 u, 16.9991 u, and 17.9992 u, respectively. The variation in atomic mass among these isotopes is due to the differences in the number of neutrons in their atomic nuclei.
Key Points
- The atomic mass of oxygen is 15.9994 u, which is a weighted average of its naturally occurring isotopes.
- Oxygen has three stable isotopes: oxygen-16, oxygen-17, and oxygen-18, with oxygen-16 being the most abundant.
- The atomic masses of oxygen-16, oxygen-17, and oxygen-18 are 15.9949 u, 16.9991 u, and 17.9992 u, respectively.
- The variation in atomic mass among oxygen isotopes is due to the differences in the number of neutrons in their atomic nuclei.
- Understanding the atomic mass of oxygen is essential for calculating the molecular masses of compounds that contain oxygen.
Isotopes of Oxygen and Their Significance
The isotopes of oxygen are significant in various fields, including chemistry, geology, and biology. Oxygen-16 is the most abundant isotope and is used as a reference for calculating the atomic masses of other elements. Oxygen-17 and oxygen-18 are used in various applications, such as in the production of radioactive isotopes for medical research and in the study of climate change.
Applications of Oxygen Isotopes
The isotopes of oxygen have several practical applications. For example, oxygen-17 is used in magnetic resonance imaging (MRI) to study the metabolism of oxygen in the brain and other tissues. Oxygen-18 is used in the study of climate change, as it can be used to determine the temperature of ancient oceans and atmospheres. Additionally, oxygen-18 is used in the production of radioactive isotopes for medical research and treatment.
| Isotope | Atomic Mass (u) | Abundance (%) |
|---|---|---|
| Oxygen-16 | 15.9949 | 99.76 |
| Oxygen-17 | 16.9991 | 0.037 |
| Oxygen-18 | 17.9992 | 0.204 |
Calculating Molecular Masses with Oxygen
Understanding the atomic mass of oxygen is essential for calculating the molecular masses of compounds that contain oxygen. The molecular mass of a compound is the sum of the atomic masses of its constituent atoms. For example, the molecular mass of water (H2O) is calculated by adding the atomic masses of two hydrogen atoms and one oxygen atom: 2(1.0079 u) + 15.9994 u = 18.0152 u.
Importance of Accurate Atomic Mass
Accurate atomic masses are crucial in various fields, including chemistry, physics, and biology. The atomic mass of oxygen is used as a reference for calculating the atomic masses of other elements. Small variations in atomic mass can significantly affect the properties and behavior of compounds, making it essential to use accurate atomic masses in calculations.
What is the atomic mass of oxygen?
+The atomic mass of oxygen is 15.9994 u, which is a weighted average of its naturally occurring isotopes.
What are the isotopes of oxygen?
+Oxygen has three stable isotopes: oxygen-16, oxygen-17, and oxygen-18, with oxygen-16 being the most abundant.
What is the significance of oxygen isotopes?
+The isotopes of oxygen are significant in various fields, including chemistry, geology, and biology, and are used in various applications, such as in the production of radioactive isotopes for medical research and in the study of climate change.
Meta description suggestion: “Learn about the atomic mass of oxygen, its isotopes, and their significance in various fields, including chemistry, geology, and biology.” (151 characters)


