Water is one of the most fascinating substances on Earth, and its freezing properties are no exception. The process of water freezing is a complex phenomenon that involves the transformation of liquid water into a solid state, known as ice. This process can occur in various ways, depending on the conditions and the presence of impurities or other substances. In this article, we will explore the different ways water can freeze, highlighting the unique characteristics and conditions of each method.
Key Points
- Supercooling: Water can be cooled below its freezing point without freezing, but it will freeze rapidly when disturbed.
- Nucleation: The presence of impurities or nucleation sites can initiate the freezing process by providing a surface for ice crystals to form.
- Homogeneous nucleation: Pure water can freeze without the need for impurities, but this process requires extremely low temperatures.
- Heterogeneous nucleation: The presence of other substances, such as dust or bacteria, can facilitate the freezing process by providing a surface for ice crystals to form.
- Flash freezing: Rapid cooling of water can result in the formation of amorphous ice, which lacks a crystalline structure.
Supercooling and Nucleation

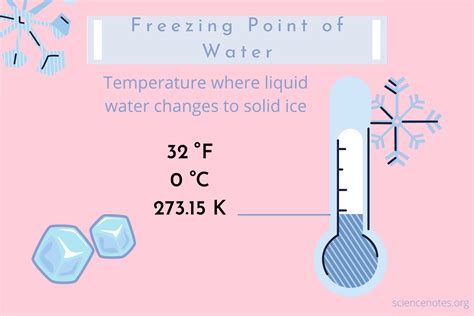
One of the most interesting ways water can freeze is through a process called supercooling. This occurs when water is cooled below its freezing point, typically around 0°C (32°F), without actually freezing. This can happen when the water is pure and free of impurities, which can act as nucleation sites for ice crystals to form. However, if the supercooled water is disturbed, it will rapidly freeze, a process known as nucleation. This is because the disturbance provides a surface for ice crystals to form, allowing the water to release its latent heat and freeze.
Nucleation Sites
Nucleation sites can be anything from dust particles to bacteria, and they play a crucial role in the freezing process. When a nucleation site is present, it provides a surface for water molecules to come together and form ice crystals. This process is facilitated by the presence of imperfections or irregularities on the surface of the nucleation site, which can help to lower the energy barrier for ice crystal formation. As a result, the freezing process can occur more easily and at a higher temperature than it would in the absence of nucleation sites.
| Freezing Method | Description |
|---|---|
| Supercooling | Water is cooled below its freezing point without freezing, but will freeze rapidly when disturbed. |
| Nucleation | The presence of impurities or nucleation sites initiates the freezing process by providing a surface for ice crystals to form. |
| Homogeneous nucleation | Pure water freezes without the need for impurities, but this process requires extremely low temperatures. |
| Heterogeneous nucleation | The presence of other substances, such as dust or bacteria, facilitates the freezing process by providing a surface for ice crystals to form. |
| Flash freezing | Rapid cooling of water results in the formation of amorphous ice, which lacks a crystalline structure. |
Homogeneous Nucleation

Another way water can freeze is through a process called homogeneous nucleation. This occurs when pure water is cooled to an extremely low temperature, typically around -40°C (-40°F), without the presence of impurities or nucleation sites. At this temperature, the water molecules are able to come together and form ice crystals spontaneously, without the need for a nucleation site. However, this process is relatively rare and requires very specific conditions, making it less common than heterogeneous nucleation.
Heterogeneous Nucleation
Heterogeneous nucleation is the most common way water freezes, and it occurs when the presence of other substances, such as dust or bacteria, facilitates the freezing process. These substances can provide a surface for ice crystals to form, allowing the water to release its latent heat and freeze. This process is important in various natural phenomena, such as the formation of ice in clouds and the freezing of lakes and rivers.
Flash Freezing
Flash freezing is a process that occurs when water is cooled rapidly, typically using a technique called vitrification. This process involves cooling the water so quickly that it does not have time to form ice crystals, resulting in the formation of amorphous ice. Amorphous ice lacks a crystalline structure and is typically more disordered than crystalline ice. Flash freezing is used in various applications, such as the preservation of biological samples and the production of ice cream.
What is the difference between supercooling and nucleation?
+Supercooling occurs when water is cooled below its freezing point without freezing, while nucleation is the process by which the water freezes rapidly when disturbed. Nucleation sites, such as impurities or imperfections, provide a surface for ice crystals to form, allowing the water to release its latent heat and freeze.
What is homogeneous nucleation?
+Homogeneous nucleation is the process by which pure water freezes without the need for impurities or nucleation sites. This process requires extremely low temperatures, typically around -40°C (-40°F), and is relatively rare.
What is the difference between heterogeneous nucleation and flash freezing?
+Heterogeneous nucleation occurs when the presence of other substances, such as dust or bacteria, facilitates the freezing process, while flash freezing occurs when water is cooled rapidly, resulting in the formation of amorphous ice. Heterogeneous nucleation is a more common process and is important in various natural phenomena, while flash freezing is used in various applications, such as the preservation of biological samples.
In conclusion, the freezing of water is a complex phenomenon that can occur in various ways, depending on the conditions and the presence of impurities or other substances. Understanding these different processes is essential for various applications, from cryopreservation to the study of water’s unique properties. By recognizing the unique characteristics and conditions of each freezing method, we can better appreciate the fascinating world of water and its many wonders.