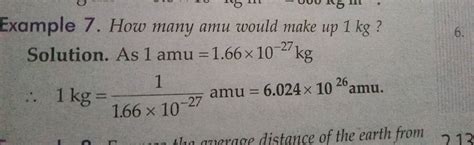The atomic mass unit (amu) to kilogram (kg) conversion is a fundamental concept in physics and chemistry, particularly when dealing with the masses of atoms and molecules. Understanding this conversion is crucial for calculating the mass of substances in various units, which is essential in both theoretical and practical applications across different fields of science.
Key Points
- The atomic mass unit (amu) is defined as one-twelfth the mass of a carbon-12 atom.
- The kilogram (kg) is the base unit of mass in the International System of Units (SI), defined as the mass of the International Prototype Kilogram.
- To convert amu to kg, we use the conversion factor where 1 amu = 1.66053904 × 10^-27 kg.
- This conversion is essential for calculating the mass of atoms, molecules, and substances in different units.
- Understanding the relationship between amu and kg helps in various scientific calculations, including those in chemistry, physics, and engineering.
Understanding the Atomic Mass Unit (amu)
The atomic mass unit, also known as the unified atomic mass unit (u or amu), is a standard unit of mass that is used to express the mass of atoms and molecules. It is defined as one-twelfth the mass of a carbon-12 atom. This definition provides a universal standard for measuring the mass of atomic and subatomic particles, facilitating comparisons and calculations across different elements and compounds.
Conversion Factor: amu to kg
The conversion from amu to kg is based on the precise definition of the amu and its relationship to the kilogram. The conversion factor is as follows: 1 amu = 1.66053904 × 10^-27 kg. This factor is derived from the definition of the amu and the known mass of the carbon-12 atom, which serves as the reference point for atomic masses.
| Unit | Conversion Factor |
|---|---|
| 1 amu | 1.66053904 × 10^-27 kg |
Practical Applications of amu to kg Conversion
The ability to convert between amu and kg is vital in various scientific and engineering applications. For instance, in chemistry, understanding the mass of molecules in both amu (for atomic and molecular-level interactions) and kg (for bulk quantities) is essential for calculating reaction stoichiometry, yields, and the physical properties of substances. In physics, particularly in nuclear and particle physics, precise mass measurements are critical for understanding fundamental interactions and properties of matter.
Calculation Example
To illustrate the conversion, consider calculating the mass in kilograms of a mole of carbon dioxide (CO2), which has a molecular mass of approximately 44 amu. First, calculate the total mass of one mole of CO2 in amu, then apply the conversion factor to find the mass in kilograms.
Given: Molecular mass of CO2 = 44 amu, Avogadro's number = 6.022 × 10^23 molecules/mole.
Mass of one mole of CO2 in amu = 44 amu/molecule * 6.022 × 10^23 molecules/mole = 2.64888 × 10^25 amu/mole.
Conversion to kg: Mass in kg = 2.64888 × 10^25 amu/mole * 1.66053904 × 10^-27 kg/amu = 44.009 kg/mole.
What is the definition of an atomic mass unit (amu)?
+The atomic mass unit (amu) is defined as one-twelfth the mass of a carbon-12 atom, serving as a standard unit for expressing the mass of atoms and molecules.
How do you convert amu to kg?
+To convert amu to kg, use the conversion factor 1 amu = 1.66053904 × 10^-27 kg. Multiply the mass in amu by this factor to obtain the mass in kilograms.
Why is the conversion between amu and kg important?
+The conversion is crucial for scientific calculations, especially in chemistry and physics, where accurate mass measurements are necessary for understanding properties and behaviors of substances at both atomic and bulk levels.
In conclusion, the conversion from amu to kg is a fundamental process that bridges the gap between the atomic and macroscopic worlds, enabling precise calculations and deep understanding of scientific principles. By mastering this conversion, scientists and engineers can better navigate the complexities of mass measurements, contributing to advancements in various fields of science and technology.