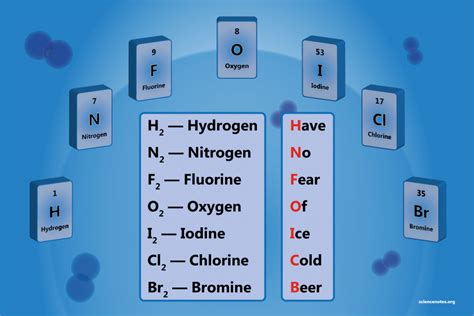
The world of chemistry is vast and intricate, with various elements that form the basis of our understanding of matter. Among these elements, diatomic elements hold a special place due to their unique properties and the fact that they exist as molecules composed of two atoms of the same element. In this article, we will delve into the realm of diatomic elements, exploring what they are, their characteristics, and the seven diatomic elements recognized in the periodic table.
Key Points
- Definition and explanation of diatomic elements
- Overview of the seven diatomic elements: Hydrogen (H2), Nitrogen (N2), Oxygen (O2), Fluorine (F2), Chlorine (Cl2), Iodine (I2), and Bromine (Br2)
- Physical and chemical properties of diatomic elements
- Importance and applications of diatomic elements in various industries
- Detailed analysis of each diatomic element, including their occurrence, extraction, and uses
Introduction to Diatomic Elements
Diatomic elements are chemical elements that exist as diatomic molecules, meaning each molecule of these elements is composed of two atoms of the same element. This characteristic distinguishes them from other elements, which may exist as monatomic (single atoms), polyatomic molecules (more than two atoms), or in other forms like networks or lattices. The diatomic elements are crucial for various biological and industrial processes, showcasing their versatility and importance in the natural world and human society.
Physical and Chemical Properties
The physical and chemical properties of diatomic elements vary significantly, reflecting their unique atomic structures and bonding characteristics. For instance, some diatomic elements like Oxygen (O2) and Nitrogen (N2) are gases at room temperature, while others like Iodine (I2) are solids. The reactivity of these elements also spans a wide range, from the highly reactive Fluorine (F2) to the relatively inert Nitrogen (N2) under standard conditions. Understanding these properties is essential for applying diatomic elements in different contexts, from biological systems to industrial applications.
| Element | Symbol | Atomic Number | Physical State at Room Temperature |
|---|---|---|---|
| Hydrogen | H2 | 1 | Gas |
| Nitrogen | N2 | 7 | Gas |
| Oxygen | O2 | 8 | Gas |
| Fluorine | F2 | 9 | Gas |
| Chlorine | Cl2 | 17 | Gas |
| Iodine | I2 | 53 | Solid |
| Bromine | Br2 | 35 | Liquid |

Detailed Analysis of Diatomic Elements

Each of the seven diatomic elements has its unique history, properties, and applications. For example, Hydrogen (H2) is the lightest and most abundant chemical element in the universe, with potential applications in clean energy, particularly as a fuel source in hydrogen fuel cells. Nitrogen (N2), making up about 78% of Earth’s atmosphere, is crucial for life as a component of amino acids, the building blocks of proteins. Oxygen (O2), essential for respiration in most living organisms, is also a critical component of the atmosphere, comprising about 21% of the air we breathe.
Occurrence, Extraction, and Uses
The occurrence, extraction, and uses of diatomic elements vary widely. Fluorine (F2), for instance, is extracted from fluorite (calcium fluoride) and is used in the production of fluoridated water, toothpaste, and certain medications. Chlorine (Cl2), often extracted from seawater, is widely used as a disinfectant in water treatment and in the production of plastics like PVC. Iodine (I2), obtained from seaweed and underground brines, is essential for the production of iodized salt, which helps prevent iodine deficiency disorders. Bromine (Br2), used in flame retardants, fumigants, and dyes, is extracted from seawater and salt lakes. Understanding the sources, extraction methods, and applications of these elements is crucial for managing resources sustainably and developing new technologies.
The applications of diatomic elements extend into various sectors, including healthcare, where Oxygen (O2) is used in life-support systems, and industry, where Nitrogen (N2) and Argon are used to create inert atmospheres for welding and semiconductor manufacturing. The role of these elements in agriculture, environmental conservation, and energy production further highlights their multifaceted importance.
What are diatomic elements, and why are they important?
+Diatomic elements are chemical elements that exist as molecules made of two atoms of the same element. They are important due to their unique properties and roles in biological, industrial, and environmental processes.
How are diatomic elements used in industry and daily life?
+Diatomic elements have a wide range of applications, from being components of the air we breathe (Oxygen and Nitrogen) to being used in water purification (Chlorine), in the production of plastics (Chlorine), and as potential clean energy sources (Hydrogen).
What challenges are associated with the extraction and use of diatomic elements?
+The extraction and use of diatomic elements can pose environmental and health challenges. For example, the extraction of Chlorine and Fluorine can have harmful effects if not managed properly, and the reactive nature of some diatomic elements requires careful handling to prevent accidents.
In conclusion, diatomic elements are a fascinating group of chemical elements that play critical roles in the natural world and human society. Their unique properties, diverse applications, and the challenges associated with their extraction and use underscore the complexity and importance of chemistry in understanding and addressing the needs of our world. As we continue to explore and apply knowledge about these elements, we are not only advancing our understanding of chemistry but also contributing to sustainable development and the well-being of future generations.